Biotechnology is generally defined as the application of living organisms to create or modify products or processes for practical purposes. Within this multidisciplinary field, different types/colors are used to group its branches according to their application sectors. In this blog, we will focus on green biotechnology, explaining what it is, its applications, how it relates to environmental biotechnology, how it differs from other types, its benefits and risks, some practical examples, and finally, a review of the various colors of biotechnology and what each one represents.
What is green biotechnology?
Green biotechnology is the branch of biotechnology focused on agricultural and environmental fields. It includes the application of biotechnological techniques to plants, crops, and terrestrial ecosystems. In other words, it encompasses all biotechnological processes applied to the agricultural sector, including genetic improvement of plants, the use of beneficial microorganisms in agriculture, and the development of biological products for the field. Given its close relationship with plant sciences, it is sometimes also referred to as plant biotechnology or agro-biotechnology. Currently, many farmers around the world use these tools to combat pests, nourish crops, and make plants more resistant to diseases and extreme weather conditions (droughts, frosts, etc.).

Cotton on the right, genetically modified, shows significantly less insect damage compared to the conventional cotton on the left. Image source: CSIRO
What are the applications of green biotechnology?
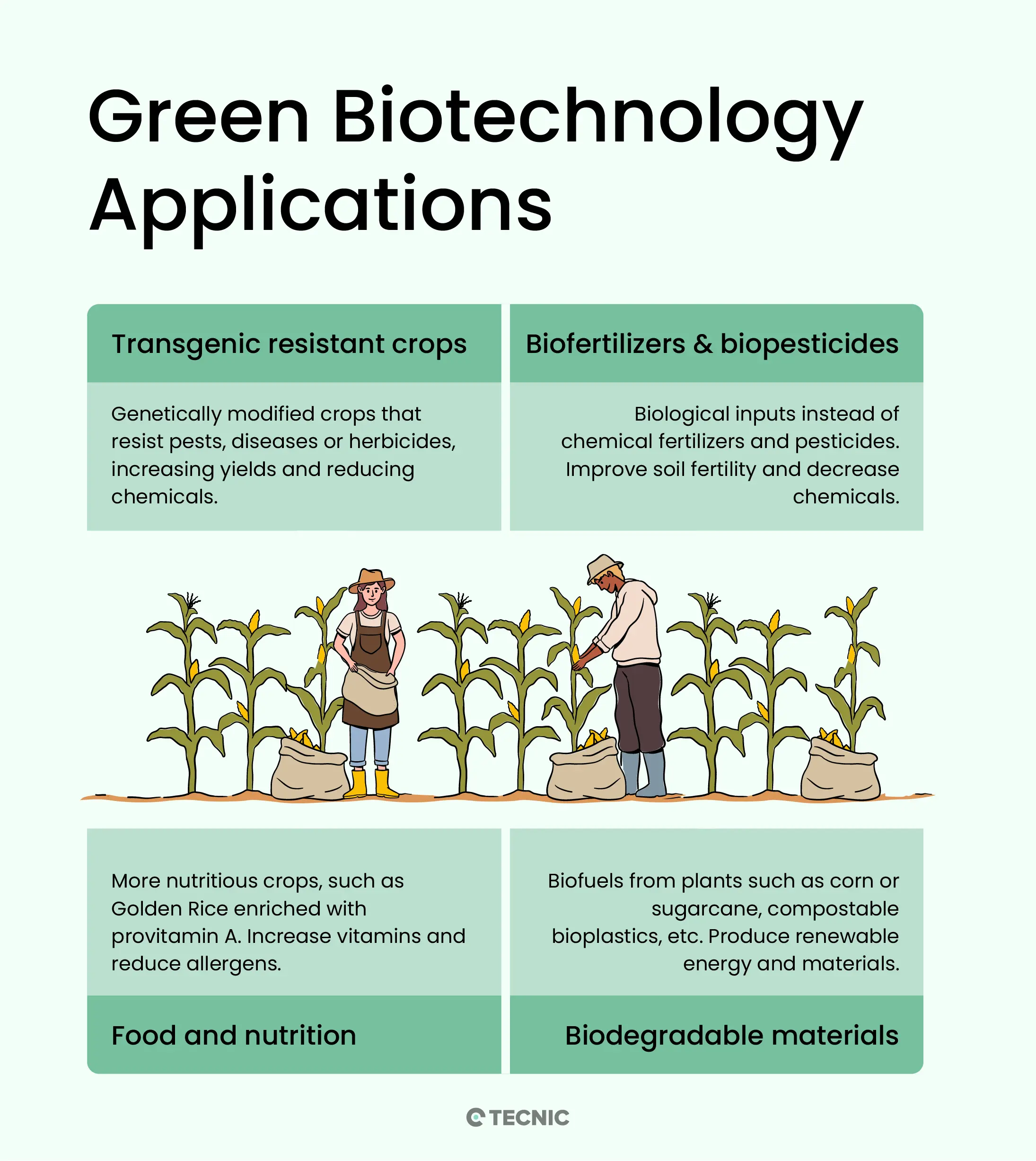
Green biotechnology has a wide range of applications today. Some of the main ones include:
- Transgenic resistant crops: Through recombinant DNA techniques, genetically modified plants resistant to pests, diseases, or herbicides have been developed. These crops (such as transgenic corn, soybeans, or cotton) have higher yields and require fewer chemical pesticides, reducing the use of agrochemicals in the field. For example, transgenic corn tolerant to glyphosate has been created, allowing farmers to control weeds without damaging the crop and reducing the amount of herbicide needed.
- Biofertilizers and biopesticides: Another application is the development of biological inputs as alternatives to chemical fertilizers and pesticides. Biofertilizers are microorganisms (such as nitrogen-fixing bacteria or mycorrhizae) that, when inoculated into seeds or soils, increase nutrient availability for plants, naturally improving soil fertility. This reduces the use of chemical fertilizers while maintaining or even enhancing long-term soil quality. Similarly, biopesticides help control insects or fungi in a targeted way, reducing the need for synthetic pesticides and their environmental impacts.
- Food and nutrition improvement: Green biotechnology also aims to obtain more nutritious and higher-quality foods. A prominent case is Golden Rice, a genetically modified rice variety that produces beta-carotene (provitamin A) in the grain, intended to combat vitamin A deficiency in populations where rice is a staple food. Additionally, oilseed crops have been modified to produce healthier vegetable oils (with less saturated fat), fruits enriched with vitamins, or crops with reduced toxin or allergen content. These improvements contribute to food security by providing healthier and more abundant food.
- Biofuels and biodegradable materials: Another major application is the production of renewable energy and sustainable materials from biomass. For example, fermenting sugars from corn or sugarcane to obtain bioethanol, or using vegetable oils (such as rapeseed or palm) to produce biodiesel, are biotechnological processes that create biofuels, reducing dependence on fossil fuels. Research is also underway into obtaining biodegradable bioplastics from corn starch or other plant-based sources. These developments, sometimes part of what is called industrial white biotechnology, are closely linked to green biotechnology when the raw material comes from plant cultivation.
Green biotechnology can also contribute to environmental restoration. For example, through phytoremediation (using plants to absorb or degrade pollutants), it is possible to decontaminate soils with heavy metals or wastewater naturally. Biotechnological processes are also used in managing organic waste, such as producing compost from agricultural residues or anaerobic digestion to generate biogas. In summary, green biotechnology applications range from achieving more efficient and ecological agriculture to producing clean energy and helping to remediate the environment.
How is it related to environmental biotechnology?
Given its focus on sustainability, green biotechnology is closely linked to what is often called environmental biotechnology. Environmental biotechnology refers to the use of microorganisms, plants, or other living beings to protect, conserve, or restore the environment. It includes techniques such as bioremediation to clean up contaminated soils, treat wastewater, control gas emissions, or recycle waste—leveraging the ability of certain organisms to degrade toxic substances. This ecosystem-focused branch is typically classified under the color grey in biotechnology, as its main goal is conserving the natural environment through biological solutions.
Green and environmental biotechnologies share the goal of achieving a more harmonious interaction between technology and nature. In fact, many “green” applications have clear environmental benefits. For example, transgenic crops resistant to insects reduce the amount of chemical insecticides released into the environment, and biofertilizers lower soil and groundwater pollution by replacing synthetic fertilizers. Some genetically modified plants can even be directly used in bioremediation (absorbing heavy metals from soil, degrading explosives, etc.), blurring the line between green and grey biotechnologies.
In short, green biotechnology includes an important environmental aspect (making agriculture more sustainable and eco-friendlier), while environmental biotechnology (grey) specializes exclusively in addressing pollution and ecological conservation problems. They are complementary fields: green biotechnology creates cleaner, more efficient farming systems, and environmental biotechnology focuses on repairing damage and maintaining healthy ecosystems.
What are the benefits and risks of green biotechnology?
As we have seen, green biotechnology has the potential to make food and resource production more sustainable, but it also presents some challenges. Below are its main benefits and associated risks or concerns:
Benefits of green biotechnology:
- Healthier and more sustainable agriculture: It enables the development of more nutritious crops (e.g., higher vitamin content) that are also safer, free from natural toxins or allergens. By creating pest- and disease-resistant plants, the use of pesticides and chemicals in fields is reduced, lessening soil and water contamination. This results in healthier food for consumers and more environmentally friendly agricultural practices.
- Increased production and food security: Genetic improvements can boost crop yields and enable cultivation under difficult conditions (poor soils, drought, salinity). This helps produce more food with less land, supporting efforts to combat hunger and poverty in vulnerable regions. For example, certain modified varieties allow farmland to produce more, particularly benefiting developing countries facing growing food demand.
Risks and challenges of green biotechnology:
- Loss of biodiversity and ecological imbalances: Widespread use of a few transgenic crops could lead to genetic erosion (loss of crop variety diversity). There are also concerns that introducing modified organisms could alter ecological interactions, affecting the balance of surrounding natural ecosystems. For example, if a transgenic plant crossbreeds with wild relatives, it could transfer competitive advantages and become an unwanted invasive species.
- Unpredictable effects on health and environment: Although all commercial biotech crops undergo safety assessments, there is always a risk of unforeseen effects. Concerns have been raised about transgenic foods potentially causing new allergies in some people, or genetically modified organisms escaping from labs and disrupting natural communities. While there is no conclusive evidence of harm to human health from consuming approved GMOs, public perception of risk remains in some sectors.
- Socioeconomic impact: The adoption of biotech technologies in agriculture may have economic and social consequences. On one hand, increased yields and automation could reduce the need for agricultural labor, affecting rural employment. On the other hand, many transgenic seeds are developed and patented by large companies, raising concerns about farmers’ dependence on these corporations and the possible exclusion of small producers unable to afford the new technologies. These issues call for regulatory frameworks and public policies to ensure that biotechnology benefits society fairly.
In summary, green biotechnology offers clear advantages, such as more productive, cleaner, and more nutritious agriculture, but also raises legitimate concerns regarding biodiversity preservation, ecological safety, public acceptance of transgenic foods, and economic fairness. A responsible approach involves evaluating these benefits and risks case by case, based on scientific evidence and solid regulations.

What are some examples of applied green biotechnology?
There are numerous examples showing how green biotechnology is currently applied in modern agriculture and beyond:
- Large-scale transgenic crops: Several countries have adopted genetically modified crops. For example, in Argentina, nearly 100% of cultivated soybeans are transgenic (herbicide-tolerant), and more than 99% of planted corn is also transgenic, often combining insect resistance and herbicide tolerance. Similarly, nearly 100% of Argentine cotton contains Bt genes (insect resistance) plus herbicide tolerance. This high adoption rate reflects farmers’ confidence in the benefits of these crops, such as reduced insecticide use, higher yields, and lower production costs.
Worldwide, countries like the United States, Brazil, India, China, Canada, and Paraguay grow millions of hectares of transgenic soy, corn, cotton, canola, and more. A notable example is Bt cotton, modified with a Bacillus thuringiensis gene to make it toxic to key pests; its introduction drastically reduced the amount of insecticides used in cotton fields, benefiting both farmers’ economics and the environment.
- Nutritional and quality improvements: As mentioned, Golden Rice is a well-known case of green biotechnology aimed at fighting vitamin A deficiency. Although its commercial adoption has been slow, it represents a milestone in using transgenic crops for humanitarian purposes. Other examples include soybeans modified to produce healthier oil (higher omega-3 fatty acids, for instance), or biofortified corn with higher levels of essential amino acids for improved animal feed. Transgenic papaya varieties resistant to the “ringspot virus” have also been developed, saving the crop from pests that devastated its production (a successful case implemented in Hawaii since the 1990s). These illustrate how genetic engineering can solve specific agricultural problems and add nutritional or commercial value to crops.
- Biofertilizers and organic agriculture: In various countries, biofertilizers are commercially available for different crops. For instance, specific Rhizobium strains are used to inoculate legume seeds (soy, beans, alfalfa), enabling them to fix atmospheric nitrogen in the soil and meet most of their nitrogen needs. Likewise, dried formulations of mycorrhizal fungi are applied in horticultural or forestry crops to enhance nutrient and water uptake by roots. These products, derived from biotechnology, allow for more “organic” agriculture by reducing reliance on synthetic chemicals. Regarding biopesticides, a common example is using Bacillus thuringiensis (or its purified toxins) to control insect larvae in crops like corn or vegetables, avoiding traditional insecticides. Specific viruses (entomopathogenic viruses) are also used as targeted bioinsecticides. These strategies have been made possible by biotechnological research that identified and multiplied such biological control agents.
- Phytoremediation and environmental conservation: Green biotechnology is applied in plant-based bioremediation projects. An experimental example is the use of sunflowers and other plant species to extract heavy metals from contaminated soils near old mines or factories (the metals concentrate in plant tissues, which are then safely harvested and disposed of). Another case is genetically modified poplar trees that degrade industrial solvents in groundwater, cleaning up polluted aquifers.
Additionally, in arid ecosystems, highly resistant plants (cacti, dry shrubs) supported by microbial biostimulants are being introduced to restore degraded lands. This relates to so-called brown biotechnology, a branch of green biotechnology adapted to desert areas. Although many of these examples are in research or pilot stages, they demonstrate green biotechnology’s versatility beyond food crops.
- Fermented food production: Traditional biotechnological techniques have been applied in food production for centuries. The making of wine, beer, bread, yogurt, cheese, and other fermented products relies on microorganisms (yeasts, bacteria) that transform basic ingredients into goods with desired characteristics. While these processes have been known since antiquity, modern biotechnology has optimized many of these fermentations. For example, yeast strains have been selected for higher efficiency or specific flavors, and purified microbial enzymes are used in the dairy industry to improve cheese ripening. All of this is part of yellow biotechnology, showing that the line between “traditional” and “modern” is sometimes blurred: an artisanal process like fermentation can be enhanced thanks to advanced biotechnological knowledge.

Golden Rice grain compared to white rice grain in screenhouse of Golden Rice plants.
What are the different colors of biotechnology and what do they represent?
In total, there are more than ten defined “colors” in biotechnology. The main ones (red, green, blue, white, yellow) were created to distinguish major areas of application, while others (grey, gold, brown, purple, orange, black) have been added for more specific fields as biotechnology continues to expand. This classification offers a broad overview of biotechnology’s potential across various sectors of society. Whether improving health, feeding us, caring for the planet, boosting industry, or exploring oceans and genetic data, biotechnology, in all its colors, plays a crucial role in the modern world and promises to be a key tool for building a more sustainable and innovative future. If you’re curious about the rest of the biotech colors, we’ve published a full blog that explores each one in depth.
Frequently Asked Questions (FAQ)
Green biotechnology applies biological techniques to agriculture and the environment, aiming to improve crops, reduce chemicals, and support sustainability.
It is used to develop pest-resistant crops, create biofertilizers and biopesticides, enhance nutritional value, and produce biofuels and biodegradable materials.
Yes. Green biotechnology overlaps with environmental biotech when applied to soil recovery, phytoremediation, or sustainable farming, though environmental biotech focuses more on pollution control.
Examples include Golden Rice, Bt cotton, nitrogen-fixing biofertilizers, biodegradable plastics from corn, and phytoremediation with sunflowers.
It improves yield, reduces chemical use, enhances food nutrition, and promotes eco-friendly farming.
Colors represent application areas: red (health), green (agriculture), blue (marine), white (industrial), yellow (food), grey (environmental), etc.
Using bacteria to clean up oil spills or plants to remove heavy metals from contaminated soil (phytoremediation) are common examples of environmental biotechnology.
References
International Rice Research Institute. (n.d.). Golden Rice Project.
United States Department of Agriculture (USDA). (n.d.). Biotechnology: Frequently Asked Questions.
International Service for the Acquisition of Agri-biotech Applications (ISAAA). (2023). Global Status of Commercialized Biotech/GM Crops: 2018.
FAO. (2021). Biotechnology in food and agriculture. Food and Agriculture Organization of the United Nations. Retrieved from
- Hefferon, K. L. (2016). Agricultural biotechnology.







