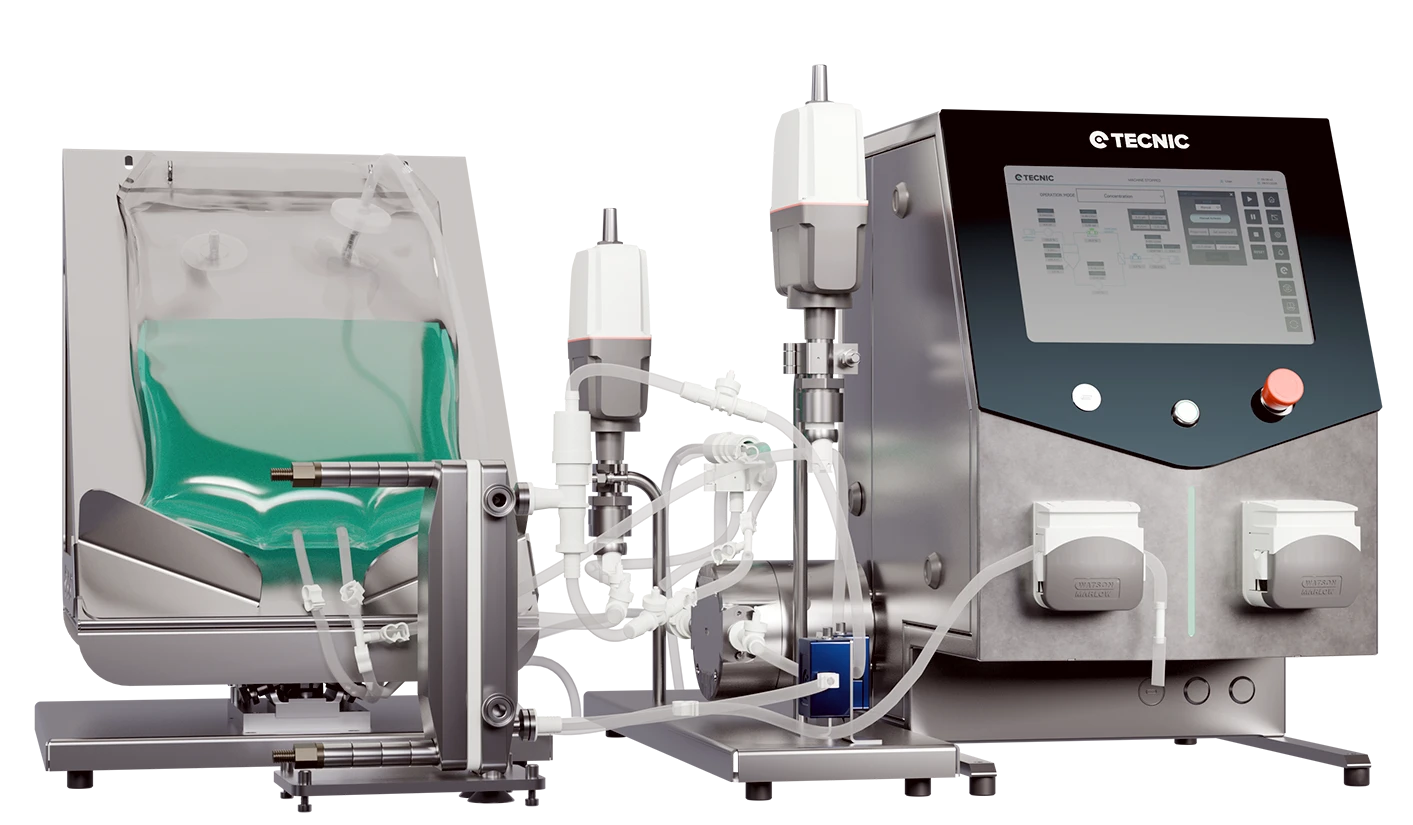
CAR-T Therapies: Reprogramming the immune system against cancer
Before diving into technical details, it's important to understand the general idea: CAR-T therapies turn the patient’s own immune defenses into a personalized treatment against cancer. A few of their T cells (the “soldiers” of the immune system) are extracted, trained in the lab by adding a “sensor” that precisely recognizes tumor cells, and once reintroduced into the body, these modified cells seek out and destroy those targets.
CAR-T therapies (Chimeric Antigen Receptor T-cell) are a form of advanced immunotherapy in which the patient’s own white blood cells (T cells) are engineered in the lab to express an artificial receptor (CAR) designed to recognize and destroy specific tumor cells.
In the standard process, each patient undergoes apheresis to obtain leukocytes, their T cells are isolated, and then genetically modified to express a chimeric antigen receptor (CAR) targeted against a tumor antigen. The CAR combines the outer part of an antibody (which recognizes the antigen on the cancer cell) with internal T-cell signaling domains (such as CD3ζ and co-stimulatory molecules like CD28 or 4-1BB). This allows T cells to “see” cancer antigens they were not previously programmed to detect, and upon binding to them, to activate and kill those malignant cells. Finally, after massive expansion in culture, millions of modified CAR-T cells are reinfused into the patient to attack the tumor.
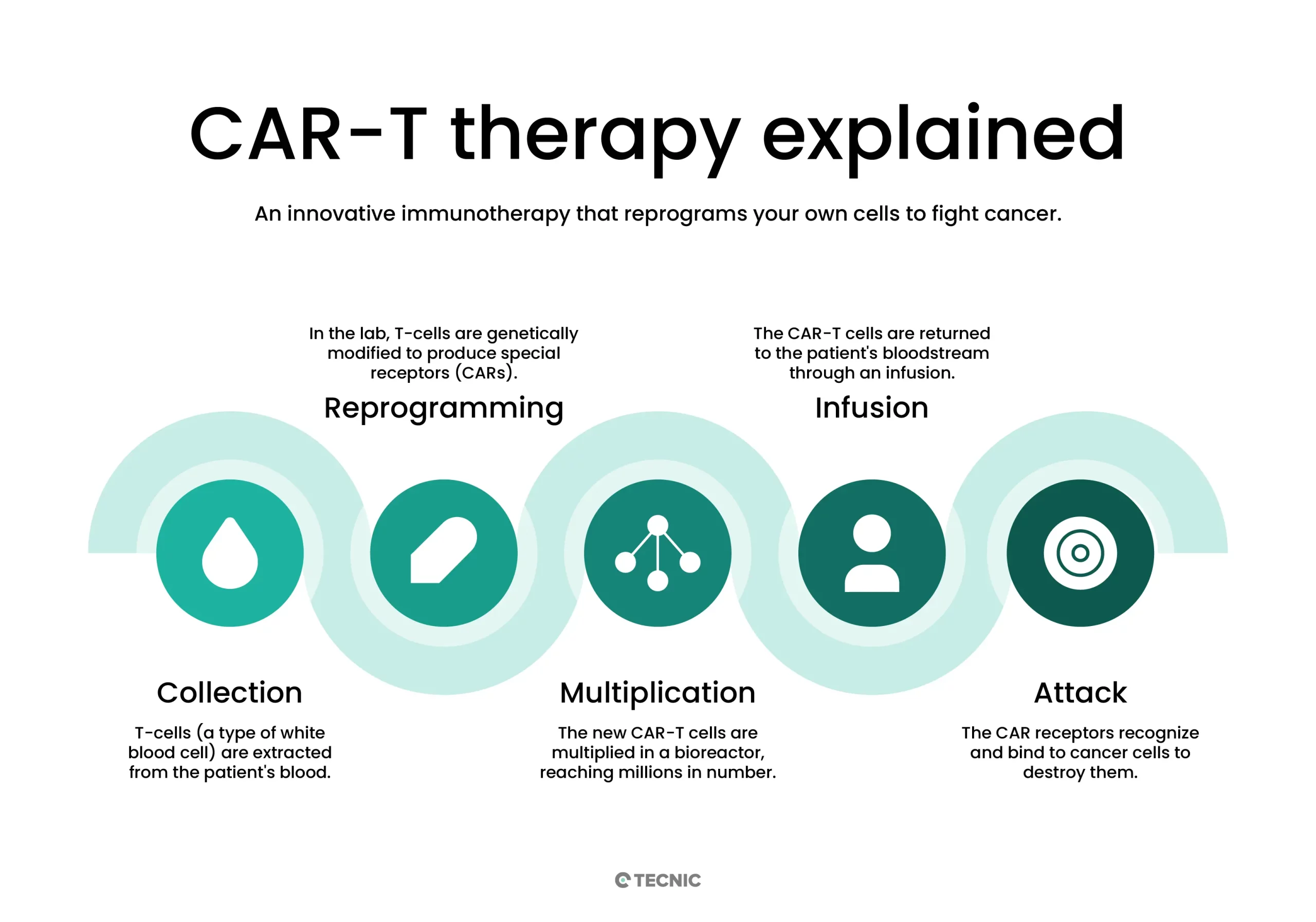
To better understand it, the CAR-T manufacturing process includes:
- Collection and Isolation: Blood is collected from the patient (leukocytes), and T cells are isolated.
- Genetic Modification: In the lab, the T cells are activated and transduced with a vector (usually viral) that introduces the gene for the specific CAR receptor. This receptor enables the T cells to recognize the desired tumor antigen.
- Cell Expansion: The modified T cells are cultured in specialized bioreactors to multiply, typically over several days (e.g., 7–10 days) until reaching the required therapeutic dose.
- Quality Control and Infusion: The final cell product is either frozen or prepared for infusion. Tests are conducted to ensure sterility, viability, CAR-T cell percentage, and other parameters. Finally, the modified cells are reinfused into the patient, where they circulate and attack the identified tumor cells.
This gene-cell hybrid process enables a highly specific therapy: the modified T cells act as a “living drug” that selectively targets cancer, minimizing damage to healthy tissues.
Current and emerging clinical applications
CAR-T therapies have been primarily applied to advanced and refractory hematologic cancers. In currently approved clinical practice, they include:
- B-cell Acute Lymphoblastic Leukemia (ALL): Used in children and young adults with relapsed or refractory ALL.
- Diffuse Large B-cell Lymphoma (DLBCL): For adults with advanced DLBCL unresponsive to other treatments.
- Mantle Cell Lymphoma: The first CAR-T therapy for this resistant lymphoma was recently approved.
- Other B-cell Lymphomas/Leukemias: Applications are being explored in follicular lymphoma, NK/T-cell lymphoma, and chronic lymphocytic leukemia (CLL) in clinical trials.
- Multiple Myeloma: The FDA approved CAR-T therapies targeting the B-cell maturation antigen (BCMA) for patients with multiple myeloma who have not responded to multiple lines of treatment.
In all these cases, the main target has been the CD19 antigen present on malignant B cells; the anti-BCMA therapy for myeloma introduced a new target antigen into the therapeutic arsenal. These indications show very high remission rates in patients with few alternatives (e.g., >80% remission in refractory pediatric ALL).
Emerging applications
Beyond blood cancers, CAR-T therapies are being explored in solid tumors (glioblastoma, melanoma, pancreatic, pelvic cancers, etc.). However, no CAR-T therapy has yet been approved for solid cancers due to efficacy challenges: the modified T cells face barriers such as poor tumor infiltration and an immunosuppressive microenvironment. Nonetheless, hundreds of ongoing trials aim to overcome these obstacles.
There are also emerging non-cancer indications. Encouraging remissions have recently been reported in severe autoimmune diseases treated with CAR-T. For example, in systemic lupus erythematosus and severe dermatomyositis, T cells have been reprogrammed against the CD19 antigen on autoreactive B cells, achieving lasting remissions. These uses are experimental but open the door to new applications (some researchers even mention plasma cell diseases or drug-resistant infections). In summary, CAR-T technology is currently effective for hematologic cancers (lymphomas and leukemias) and is expanding as a potential strategy for solid tumors and other diseases.
Challenges in CAR-T manufacturing and scaling
CAR-T manufacturing faces multiple technical and logistical challenges:
- Extreme Personalization (Autologous): Each CAR-T dose is unique to one patient. This means there is no mass production like conventional drugs, but rather individualized culturing per patient.
- Biological Variability: The quality and quantity of T cells extracted vary between patients (age, disease status, prior treatments). This initial variability complicates standardization. For instance, the presence of monocytes or suppressor cells in the sample can hinder T cell expansion.
- Technical Complexity: Multiple steps are required (isolation, activation, transduction, expansion, washing, etc.) in tightly controlled environments. The use of viral vectors (most commonly used to insert the CAR gene) is efficient but demands rigorous preclinical safety and GMP testing, increasing development time and cost.
- High Costs: Equipment, reagents, and specialized personnel are very expensive. The cost of a single CAR-T treatment can be tens or hundreds of thousands of dollars, excluding hospitalization and side effect management. Some analyses estimate the total cost (product plus hospitalization) can exceed one million dollars per patient.
- Manufacturing Time: The entire process usually takes several weeks from collection to reinfusion. During this time, the cells must remain viable and functional. The delay creates a critical period in which the patient must remain stable while their cells are cultured.
- Complex Logistics: It is necessary to coordinate the patient’s blood apheresis, transport to the manufacturing facility, lab capacity, and then return the product to the hospital. For example, fresh cells only have a short viability window for culturing. The timeline is tight: extraction, culturing, and infusion must be synchronized to avoid product expiration.
- Regulatory Requirements: Production requires cleanroom facilities (GMP), specialized equipment (clinical bioreactors, monitors, etc.), and highly trained personnel in cell therapy. Each batch must undergo exhaustive quality control (sterility, endotoxins, CAR-T cell percentage, cytotoxic activity, etc.), adding time and resources. Moreover, regulatory agencies (FDA, EMA) demand complete process validation and compliance with strict standards in both the U.S. and EU. Collectively, these factors greatly increase manufacturing costs and create bottlenecks to scale the therapy.
In summary, the key challenges in CAR-T manufacturing are poor scalability (one patient per process), biological material variability, long production cycles, and high cost/regulatory burden. These obstacles make it difficult to expand the therapy from limited use to broad access.
Technological solutions for production and scale-up
In response to these challenges, the biotech industry is advancing with technologies designed to automate, standardize, and accelerate CAR-T manufacturing:
- Automated and Closed Systems: Integrated platforms that perform several steps (isolation, activation, transduction, expansion) in a single closed system minimize manual handling and contamination risk. For example, automated processors (similar to clinical platforms) allow the generation of CAR-T batches using standardized workflows. These technologies streamline the process and improve reproducibility, facilitating scale-up.
- Bioreactors: Next-generation cell culture bioreactors are used, such as gas-permeable bag systems or stirred bioreactors, enabling high-density T cell growth. These units are equipped with sensors (pH, oxygen) and allow nutrient recirculation, so cells can expand without continuous intervention. This reduces manual handling and increases cell yield.
- Microfluidics and Miniaturization: New developments use microfluidic chip-type devices to produce CAR-T cells in very small spaces with a high degree of automation. For example, a closed micro-bioreactor system has successfully generated viable clinical doses using very few reagents. This approach promises to enable point-of-care manufacturing in hospitals and reduce costs.
- Monitoring and Quality by Design: Software and in-line monitoring sensors are integrated to control critical parameters (pH, pO₂, cell proliferation) in real time. Additionally, “Quality by Design” strategies are adopted: this involves designing manufacturing protocols based on thorough characterization of critical attributes and risk analysis, ensuring a robust and reproducible process. Using process simulations and advanced statistics, engineers can optimize media and conditions, reducing batch-to-batch variability.
- Certified Reagents and Trained Personnel: Clinical-grade (GMP) certified reagents and standardized control systems are used. Automated controls (e.g., cytometry to confirm CAR expression) are incorporated to ensure quality. GMP training for personnel is essential to minimize human error.
- New Gene Transfer Techniques: To avoid the limitations of viral vectors, non-viral methods (such as electroporation or transposon systems) are being investigated to introduce the CAR gene into T cells. These techniques may shorten processing times and eliminate risks associated with viral vectors.
- Decentralized Manufacturing: As a complementary approach, decentralized models are proposed where small regional centers (with modular GMP labs) manufacture CAR-T cells locally. This would reduce logistical delays and make therapy more accessible in different regions.
Each of these technological solutions addresses specific challenges: automation and closed systems reduce human handling; advanced bioreactors and chips improve culture scalability; and strict quality controls with design-by-quality ensure consistent products. Together, the use of process engineering and automated tools paves the way for CAR-T manufacturing to become faster, safer, and more cost-effective.

Frequently Asked Questions (FAQ)
It is an advanced immunotherapy where T cells are modified to recognize and kill cancer cells.
Through apheresis, a process that separates white blood cells from the patient's blood.
CAR stands for Chimeric Antigen Receptor, a synthetic protein that helps T cells detect cancer.
They are genetically modified to express CARs and multiplied in a bioreactor.
They are reinfused into the patient’s bloodstream through an intravenous infusion.
The CAR-T cells circulate and attack cancer cells by recognizing specific antigens.
No, it's also being explored in autoimmune diseases like lupus and other conditions.
Typically, the full process takes a few weeks from cell collection to reinfusion.